Covid Check-in: Cuba's Homegrown Vaccines
Covid Check-in: Cuba's Homegrown Vaccines
With two vaccines in the final trial phase, Cuba plans to ramp up domestic and international distribution.
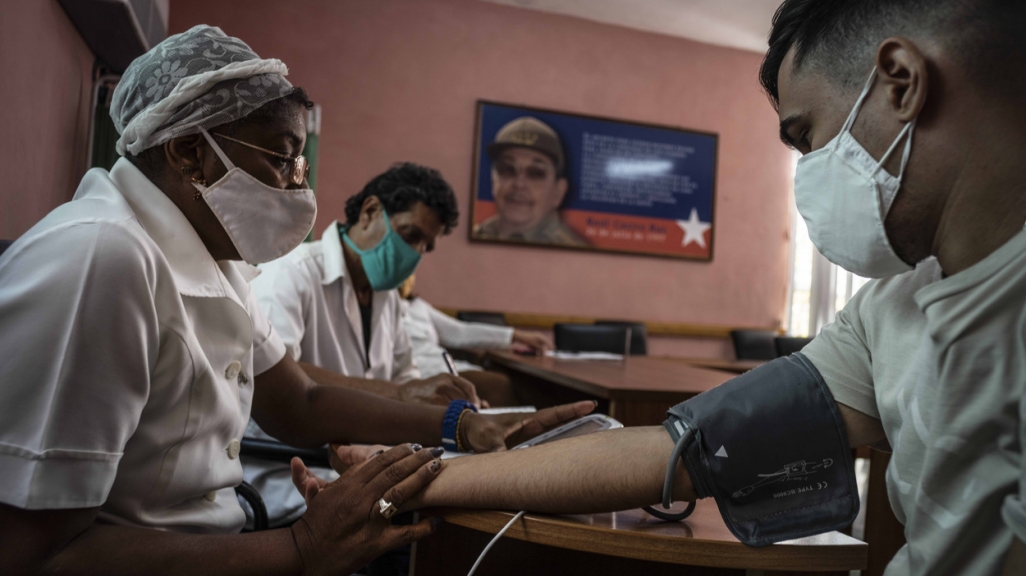
Since early in the pandemic, Cuba has been working toward producing its own vaccines. The island remains isolated from the international system due to the U.S. embargo and is not partaking in global vaccine forums like COVAX. Now the country, which has produced most of its own vaccines since the 1980s, appears to be on the verge of embarking on its immunization rollout and is beginning to vaccinate segments of its population with two of its own vaccines, each in the final trial phase.
At the start of the pandemic, Cuba began testing four vaccines: Soberana 01 (or “Sovereign 02”), Soberana 02, Abdala, and Mambisa. Both Soberana 02 and Abdala have begun Phase 3 trials, the final step before a vaccine is considered for approval by national and international health agencies. Soberana 01 is in Phase 2. Mambisa, administered nasally, is still in Phase 1. All these candidates are protein vaccines that require two doses, although a booster known as Soberana Plus is being tested as a potential third dose for Soberana 02.
AS/COA Online covers major developments and Covid-19 vaccine rollouts as countries strive to return to normalcy.
Get resources on government responses, vaccine rollouts, and health impacts in the region.